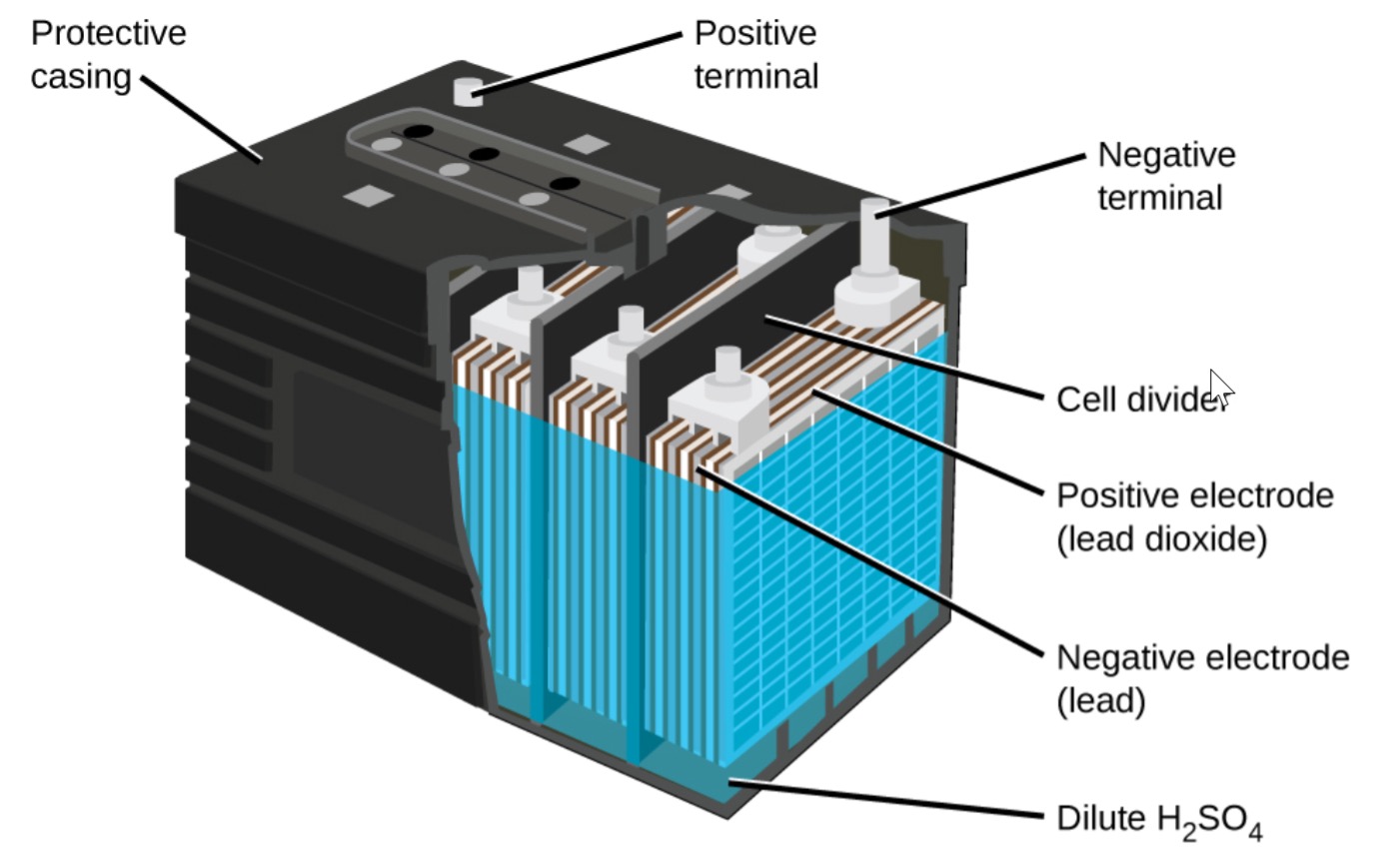
Differences, advantages, and disadvantages of batteries included in the lead acid battery family
Lead acid batteries, invented by the French physicist Gaston Planté in 1859, were the first rechargeable batteries available for commercial use. Although its age has passed, lead continues to be widely used in chemistry today. There are reasons why it is so popular; lead acid is reliable and offers a very reasonable cost per watt. Few battery types can deliver great power as cheaply as lead acid, making them cost-effective in many uses such as automobiles, golf carts, forklifts, yachts, and uninterruptible power supplies (UPS).
The grid structure of lead acid batteries is made of lead alloy. Since pure lead is very soft, it disperses easily on its own and is not suitable for use. Therefore, small amounts of other metals are added to obtain mechanical strength, thereby improving its electrical properties. The most commonly used additives are antimony, calcium, tin, and selenium. These batteries are also commonly known as "lead-antimony" and "lead-calcium".
The addition of antimony and tin improves the deep cycle, but this increases water consumption, exacerbating the need for balancing. Calcium, on the other hand, reduces self-discharge, but when overcharged, the positive lead-calcium plate has the opposite side effect due to oxidation on the grid. In the production of modern lead acid batteries, it is aimed to reduce the content of antimony and calcium by making use of additives such as selenium, cadmium, tin, and arsenic.
Lead acid is heavy and less resistant to deep cycle charging than nickel and lithium-based systems. A complete discharge causes strain on the battery and with each charge/discharge cycle, the battery permanently loses a small amount of its capacity. This dissipation is small when the battery is operated in good conditions, but the derating increases when performance drops to levels half the rated capacity. This wear characteristic applies to all battery types to varying degrees.
Depending on how deep the discharge occurs, lead acid will provide 200-300 charge/discharge cycles in deep-cycled areas. The main reason for this relatively short life cycle is the wear on the grid on the positive electrode and the consequent reduction of the active substance and the expansion of the positive plates. This wear is accelerated at high operating temperatures and when a high current is drawn.
Charging a lead acid-type battery is easy, but care must be taken with the correct voltage. By choosing a lower voltage limit, the battery is more protected, but with poor efficiency and a build-up of sulfates on the negative plate. When the high voltage limit is selected, the performance increases, but this time there is wear on the positive plate. While the sulfation process can be reversed with timely maintenance, wear is permanent.
Lead acid batteries are not well suited for rapid charging, and for most types, a full charge takes about 14-16 hours. The battery should always be kept fully charged. Low charge leads to sulfation, a condition that erodes the efficiency of the battery. This problem can be reduced by adding carbon to the negative electrode, but this reduces the specific energy level.
Lead-acid batteries have a moderate lifespan, but are not subject to memory effect, as are nickel-based systems, and have the best charge retention of all rechargeable batteries. Nickel cadmium loses about 40 percent of the energy it has stored in three months, while lead-acid loses the same amount of energy when stored for a year. When operated in sub-zero temperatures, lead-acid batteries are superior to lithium-ions and work well in cold environments.
Sealed lead acid batteries
The first sealed, or maintenance-free, lead-acid battery appeared in the mid-1970s. Engineers at the time argued that the term "sealed lead acid" was a misnomer because, they believed, no lead acid battery could be completely sealed. Valves are placed to control the ventilation both during charging, which puts pressure on the battery and during rapid discharge so that if gas accumulates in it, it can be discharged. Rather than immersing the plates in a liquid, the electrolyte is left in a moistened reagent, a design similar to nickel and lithium-based systems. This process allows the battery to operate in any physical environment without leakage.
The sealed battery contains relatively less electrolyte than the liquid-enclosed types, hence the term “acid-free”. Perhaps the most important advantage of sealed lead acid batteries is that they prevent drying out during the cycle and combine hydrogen and oxygen to release water. Here coalescence occurs at a moderate pressure of 0.14 bar (2psi). The valve acts as a safety valve against gas build-up. Repeated airing should be avoided, as in such a case drying will occur eventually.
Various types of sealed lead acid batteries have been introduced, the most common being gel, also known as valve-regulated lead acid (VRLA) and absorbent separator seal (AGM). The gel cell contains a silica-type cell that suspends the electrolyte in a paste. Smaller packages up to a capacity rating of 30Ah are more commonly known as SLA (sealed lead acid). These batteries, placed in a plastic container, are often used for small UPS, emergency lights, and wheelchairs. Due to their low price, reliability, and low maintenance requirements, SLA-type batteries continue to be preferred in hospitals, the healthcare sector, and elderly care homes. Larger VRLA batteries are used as power backup in mobile communication signal towers, internet network distribution centers, banks, hospitals, airports, and many more.
In AGM-type batteries, the electrolyte is suspended in a specially designed absorbent mat. This design offers many advantages in lead acid systems, such as faster charging and instant high current loading when desired. AGM serves best as a mid-range battery mostly in the 30 to 100Ah range and is not very suitable for large systems such as UPS. Typically, starter batteries for motorcycles are used for engine start-stop function for mini-hybrid cars as well as yachts and some vehicles that require pedaling.
Over the course of the charge cycle and with time, there is a gradual erosion of the capacity of AGM batteries; On the other hand, the performance of gel batteries is in the form of a dome curve and exhibits high performance for a long time, but its performance drops sharply towards the end of its life. AGM-type batteries are more expensive than floating-type batteries, but they are cheaper than gels. (The use of gel batteries in automobile starter batteries and engine start-stops would be very expensive.)
Unlike liquid-containing batteries, sealed lead-acid batteries are designed to have a low overvoltage to prevent the battery from reaching its potential to produce gas during charging. Overcharging produces gas, which then causes air to escape, water reduction, and ultimately dries out. As a result, the gel and partial AGM cannot be charged to full capacity and the charge voltage limit must be set lower than for batteries with liquids. This also applies in the case of a floating charge to compensate for any decrease in the battery's standby capacity after a full charge. As for charging, gel and AGM-type batteries are not a direct alternative to batteries containing liquids. If there is no low-voltage charger specifically designed for AGM-type batteries, unplug the charger after 24 hours of charging. In this way, gas output caused by a floating voltage set too high is prevented.
The optimum operating temperature for VRLA batteries is 25°C; Every 8°C increase in this temperature threshold cuts the battery life in half. Lead acid batteries are measured at discharge rates of 5 hours (0.2C) and 20 hours (0.05C). Battery performance is best when discharge is slow; capacitance measurements are significantly higher during slow discharge times. However, lead acid batteries can deliver various C currents for only a few seconds when pulsed charged. This property makes lead acid batteries a very suitable candidate as a starter battery, also known as a light-starter-starter battery (SLI). Due to their high lead and sulfuric acid content, lead acid batteries are not considered environmentally friendly.
Lead acid batteries are commonly used in three areas: Automobile (Starter battery or SLI), motive power (traction or deep cycle), and onboard (UPS).